|
血蓝蛋白(KLH)来源于大锁孔帽贝(透孔螺)的血淋巴,也被叫做匙孔血蓝蛋白,是具有高度免疫原性的蛋白大分子,也是一种多功能蛋白,它不仅具有输氧功能,而且还与能量的贮存、渗透压的维持及蜕皮过程的调节有关,还具有酚氧化物酶活性和抗菌功能,被认为是节肢动物和软体动物中的一种重要的免疫分子。
KLH广泛作为载体蛋白应用,交联于半抗原和其他抗原,使它们具有更强的免疫原性以用于抗体的制备。由于其质量大,复杂性高,与其他载体蛋白相比KLH会引出更强的免疫应答。因为此蛋白来源于软体动物,它在系统发生上远离哺乳动物物种,在测定过程中产生会与典型靶点样品发生交叉反应的抗体的可能性较小。作为大的蛋白质,KLH含有上百个伯胺和羧基基团可以成为与戊二醛,N-羟基琥珀酰亚胺酯和EDC等交联试剂交联的靶点。
传统冻干粉状态血蓝蛋白(KLH)会产生大量沉淀并可能导致在使用该产品过滤沉淀时损失高达30%-40%, 好的血蓝蛋白(KLH)制剂溶液应在结合前极少沉淀,从而可产生高效的偶联和增强抗体反应。
高分子量的天然KLH在近些年中得到了广泛的应用。但是大多数可获得的KLH配方并不适合在人体临床试验中使用。通常这些产品中的内毒素含量很高,因为血淋巴血清的生产是从冷冻动物中提取来完成的,在冻干KLH过程中可能导致沉淀产生。部分文献表明,冷冻干燥天然KLH都会导致活性的丧失。
为了KLH产品的安全性、质量和一致性,Biosyn开发了非致命性血淋巴血清采集程序,并制定了必要的动物处理和检疫技术,制定了这种关键原料规模生产的质量控制方法,在磷酸盐缓冲液中生产低内毒素和高浓度天然KLH。科研级别Native KLH是最常用的免疫载体蛋白,适用于生物活性测定、免疫学研究;同时也可用于制备多肽抗原,以进行免疫和抗体生产,疫苗产品开发等项目。
与同类产品相比,biosyn公司KLH产品具有以下特点:
独有的捕获和处理活动物的流程,不使用冷冻动物等致命提取方法,有利于KLH分子保持稳定。
在缓冲液体系中配制,比其他冻干粉产品具有更好的溶解性。
诊断级别KLH严格通过生物安全性和包括甲乙丙型肝炎、弧菌、轮状病毒、诺沃克病毒在内的无病毒检测。
高分子量,高免疫原性(400kDa)
Biosyn公司于1995年创立于美国加州,是一家专业研发和生产高质量的科研和临床级别血蓝蛋白的生物医药公司。目前biosyn公司生产的血蓝蛋白在大型国际医药公司中有约30%的市场占有率。biosyn公司的GMP级产品IMMUCOTHEL®率先于1997获得荷兰相关批文,而后也相继被韩国、阿根廷、奥地利批准进入市场。biosyn公司另一产品VACMUNE®也已于2007年向美国FDA提交了DMF文件。
.jpg)
biosyn公司在海水中养殖的软体动物大锁孔帽贝;在GMP级别厂房生产的KLH能提供极好的免疫原性,以及出色的稳定性和溶解性。
KLH产品部分文献:
Henry Milgrom, Int Arch Allergy Immunol. 2012 Feb; 157(3): 269–274. 2011 Oct 28. PMID: 22042247.
Federico Baruffaldi,Mol Pharm. 2018 Nov 5; 15(11): 4947–4962. PMID: 30240216.
Ashwin Swaminathan, Br J Clin Pharmacol. 2014 Nov; 78(5): 1135–1142. PMID: 24833186.
Jeffrey S.Miller. Clinical Immunology. Volume 117, Issue 2, November 2005, P144-151.
Brendan D. Curti, Cancer Research. December 2013.
Rong Shi PhD Marek Honczarenko MD, PhD. Clinical Pharmacology. 12 July 2016.
Willemijn Hobo, Frans Maas, Blood 2010 116:4501-4511.
Michal Radomski, Herbert J. Zeh. Journal for ImmunoTherapy of Cancer. 19 April 2016.
Mahdi Saghari, Br J Clin Pharmacol. 2021 Apr; 87(4): 1953–1962. PMID: 33025648.
Bethany Crouse,et. al. J Immunol. 2023 May 1;210(9):1272–1280. PMID: 36939374
Filipa Lemos,et. al. iScience. 2025 Mar 18;28(4):112248. PMID: 40241760
KLH: Keyhole Limpet Hemocyanin
The hemocyanin produced from the hemolymph sera of the marine mollusk Giant Keyhole Limpet, Megathura crenulata, has been in use and marketed as a crude or partially purified product for over 40 years by some chemical companies. This molecule is well recognized and commonly abbreviated as KLH. The crude research grade KLH is used in antibody production in animals against antigens. The animal sera containing the antibody for the antigen are further processed and used as immunological reagents or in immunological assays. The partially purified product has also been used in early human trials for immune status evaluation.
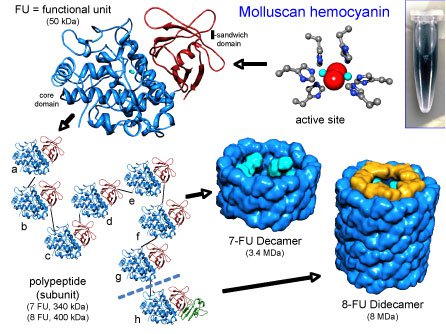
KLH structure
The native KLH is a cylindrical copper containing blue protein with a molecular mass ranging from 8-32 MDa (Million Dalton). Electron microscopy structural studies reveal presence of two oligomeric forms:
Didecamers with a molecular mass of 8 MDa, are present in the form of hollow cylinders. They are approximately 35 nm in diameter and 40 nm in length.
Multidecamers with a molecular mass of 12-32 MDa, present as stacked decamers (multidecamers) of varying length, added on one or both sides of a "nucleating" didecamer. Single decamers exhibit rectangular side views.
Keyhole Limpet Hemocyanin Structure
Prof. Dr. Jürgen Markl, Direktor Zoologisches Institut der Johannes Gutenberg-Universität Mainz, Germany
The didecamer consists of 20 subunits and occurs in two different Isoforms named KLH1 and KLH2. The subunits are biochemically and immunologically distinct. Analysis of KLH by native-PAGE gives two characteristic bands, one corresponding to KLH1 with an apparent molecular mass of about 390 kDa (kilo Dalton) and the other corresponding to KLH2 with an apparent molecular mass of 350 kDa.
biosyn native KLH, low enodoxin content
The high molecular weight, native KLH, is extensively used and has been used over the last several years. Native KLH has been manufactured and supplied by some chemical suppliers.
However, most available KLH formulations are not of the required quality and consistency suitable for use in human clinical trials. Typically, the endotoxin content in such products is very high as the hemolymph sera production is done by cutting open the animals or extraction from frozen animals. The product characteristics are also not very good and could lead to precipitation during reconstitution of lyophilized KLH. It is well documented that either freezing or lyophilization of native KLH leads to loss in activity.
Characterization of native KLH is also an issue as the large molecular weight of 8-32 MDa does not allow use of standard biochemical methods for routine quality control, which is further complicated by the use of excessive metal ions like calcium and magnesium, presumably to stabilize the molecule.
Right from the start of the hemocyanin development work in 1985, biosyn had identified these to be critical issues and developed appropriate strategies to overcome these limitations. To achieve the desired safety, quality and consistency of native KLH, biosyn has developed the non-lethal hemolymph sera collection procedure from live animals and instituted necessary animal handling and quarantine techniques. Quality control methodologies for routine production of this critical raw material has been developed.
The end result of these technology developments in the animal handling, bleeding and manufacturing process was the production of biosyn low endotoxin and high concentration native KLH of consistent high quality in a phosphate buffer.
The biosyn native KLH is supplied as High Purity Grade and Research Grade (no endotoxin testing). The GMP grade material is ideally suited for use as a carrier protein in the manufacture of vaccines for human use. It is tested for biological safety, viral clearance and heavy metal ions.
The Research Grade material is suited for use in vaccine product development activities and also for routine immunological studies, antibody production, production of activated KLH and other developmental activities. The native KLH formulations currently available from biosyn are listed in the table.
| Product |
Grade |
Uses |
| Native KLH in Phosphate buffer low endotoxin, tested for biological safety, viral clearance and metal ions |
GMP
Clinical
grade |
For further processing in KLH-conjugate vaccine manufacture for human and animal use |
| Native KLH in Phosphate buffer, or other buffers low endotoxin |
RG |
Routine immunological and antibody studies and antibody production, initial product development activity for conjugate vaccine. Preparation of Research Grade activated KLH |
| Native KLH in Phosphate buffer or other buffers. No endotoxin testing |
RG |
Low cost research grade material for research uses |
RG = Research Grade
Why use biosyn KLH?
biosyn KLH has many advantages over other commercial grade material that can be procured from other vendors. The major distinction lies in biosyn's:
Environmentally friendly process used in the extraction of hemocyanin from animals.
biosyn returns the animals back to its natural habitat, as it is a humane approach as well ensures that the animals recovers readily and more impotantly assures the product quality and reproducibility leading to no potential undefined, difficult to characterize, structural alterations to the hemocyanin molecule.
biosyn uses a proprietary methodology and has built-in a test procedure for harvesting and handling of live animals, unlike the use of dead or frozen animals or lethal extraction procedure used by some commercial KLH suppliers.
biosyn hemocynin and final products are therefore of high purity and low endotoxin quality.
biosyn KLH subunits, immunocyanin, are of low molecular weight (400 kDa) and are a completely characterized product.
biosyn manufactures and packages KLH products to meet specific customer requirements including userfriendly, ready-to-use preparation and packaging.
biosyn hemocyanin and immunocyanin are formulated in either pure buffer systems like phosphate buffer or in water and packaged as small aliquots or bulk quantities. This offers customers major advantages, i.e., flexibility of use, choice of buffer in early developmental studies, and avoidance of issues associated with reconstitution of dry powder product obtained from other vendors.
biosyn Immunocyanin in water is a unique formulation and overcomes one of the major drawbacks of using KLH in clinical and commercial grade conjugate vaccine products.
biosyn clinical grade products are extensive tested for safety and lack of viruses including hepatitis A, B, C, vibrio species, rota virus and Norwalk viruses.
|

